Helium is one of the few gases that is lighter than air. More importantly, it is quite stable, colorless, odorless and harmless, so it is a very good choice to use it to blow up self-floating balloons.
Now helium is often called “gas rare earth” or “golden gas”. Helium is often considered to be the only truly non-renewable natural resource on Earth. The more you use, the less you have, and it has a wide range of uses.
So, the interesting question is, what is helium used for and why is it non-renewable?
Where does the earth’s helium come from?
Helium ranks second in the periodic table. In fact, it is also the second most abundant element in the universe, second only to hydrogen, but helium is indeed very rare on Earth.
This is because helium has a valence of zero and does not undergo chemical reactions under all normal conditions. It usually only exists in the form of helium (He) and its isotope gases.
At the same time, because it is very light, once it appears on the surface of the earth in gas form, it will easily escape into space instead of remaining on the earth. After hundreds of millions of years of escape, there is very little helium left on Earth, but the current concentration of helium in the atmosphere can still be maintained at around 5.2 parts per million.
This is because the Earth’s lithosphere will continue to produce helium to make up for its escape loss. As we mentioned earlier, helium usually does not undergo chemical reactions, so how is it produced?
Most of the helium on Earth is the product of radioactive decay, mainly the decay of uranium and thorium. This is also the only way to produce helium at present. We cannot produce helium artificially through chemical reactions. Most of the helium formed by natural decay will enter the atmosphere, maintaining the helium concentration while continuously losing, but some of it will be locked by the lithosphere. Those locked helium are usually mixed in natural gas, and eventually developed and separated by humans.
What is helium used for?
Helium has extremely low solubility and high thermal conductivity. These characteristics allow it to be used in many fields, such as welding, pressurization and purging, which all like to use helium.
However, what really makes helium the “golden gas” is its low boiling point. The critical temperature and boiling point of liquid helium are 5.20K and 4.125K respectively, which are close to absolute zero and the lowest among all substances.
This makes liquid helium widely used in cryogenics and cooling of superconductors.
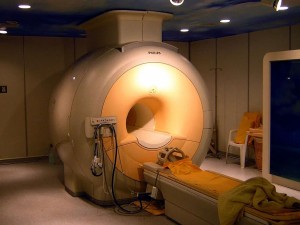
Some substances will show superconductivity at the temperature of liquid nitrogen, but some substances require lower temperatures. They need to use liquid helium and cannot be replaced. For example, the superconducting materials used in magnetic resonance imaging equipment and the European Large Hadron Collider are all cooled by liquid helium.
Our company is considering entering the liquid helium field, please stay tuned.
Post time: Aug-22-2024